
Route of administration
Source:vignettes/articles/route_of_administration.Rmd
route_of_administration.RmdIntroduction
The Caldès 2009 ganciclovir model (https://doi.org/10.1128/aac.00085-09) is capable of describing the pharmacokinetics of either injectable ganciclovir or oral valganciclovir.
mod_ganciclovir_Caldes_2009 <- function() {
ini({
THETA_cl <- 7.49
THETA_v1 <- 31.90
THETA_cld <- 10.20
THETA_v2 <- 32.0
THETA_ka <- 0.895
THETA_baf <- 0.825
ETA_cl ~ 0.107
ETA_v1 ~ 0.227
ETA_ka ~ 0.464
ETA_baf ~ 0.049
add.sd <- 0.465
prop.sd <- 0.143
})
model({
TVcl = THETA_cl*(ClCr/57);
TVv1 = THETA_v1;
TVcld = THETA_cld;
TVv2 = THETA_v2;
TVka = THETA_ka;
TVbaf = THETA_baf;
cl = TVcl*exp(ETA_cl);
v1 = TVv1*exp(ETA_v1);
cld = TVcld;
v2 = TVv2;
ka = TVka*exp(ETA_ka);
baf = TVbaf*exp(ETA_baf);
k10 = cl/v1;
k12 = cld / v1;
k21 = cld / v2;
Cc = centr/v1;
d/dt(depot) = -ka*depot
d/dt(centr) = ka*depot - k10*centr - k12*centr + k21*periph;
d/dt(periph) = k12*centr - k21*periph;
d/dt(AUC) = Cc;
f(depot)=baf;
alag(depot)=0.382;
Cc ~ add(add.sd) + prop(prop.sd) + combined1()
})
}
mod_ganciclovir_Caldes_2009 <- mod_ganciclovir_Caldes_2009()Intravenous ganciclovir
Patient record with TDM data
To describe intravenous administration, a CMT column has been added to the TDM data table to indicate administrations directly into the central compartment.
Note: to compute the AUC between the last dose and the time of the last dose + 24 hours, a dummy dose of 0 mg is added to the time of the last observation of interest (i.e. H144).
patient <- data.frame(ID=1,TIME=c(0,121,122,126,144),
DV=c(NA,10.8,5.8,3.3,NA),
ADDL=c(5,0,0,0,0),
II=c(24,0,0,0,0),
EVID=c(1,0,0,0,1),
CMT=c("centr",NA,NA,NA,"centr"),
AMT=c(250,0,0,0,0),
DUR=c(0.5,NA,NA,NA,NA),
ClCr=25)
patient
#> ID TIME DV ADDL II EVID CMT AMT DUR ClCr
#> 1 1 0 NA 5 24 1 centr 250 0.5 25
#> 2 1 121 10.8 0 0 0 <NA> 0 NA 25
#> 3 1 122 5.8 0 0 0 <NA> 0 NA 25
#> 4 1 126 3.3 0 0 0 <NA> 0 NA 25
#> 5 1 144 NA 0 0 1 centr 0 NA 25Individual PK profile and AUC 0-24
The individual PK profile can be estimated, and plotted.
map_patient <- poso_estim_map(patient,mod_ganciclovir_Caldes_2009)
#> using C compiler: ‘gcc (Ubuntu 13.3.0-6ubuntu2~24.04) 13.3.0’
plot(map_patient$model,Cc)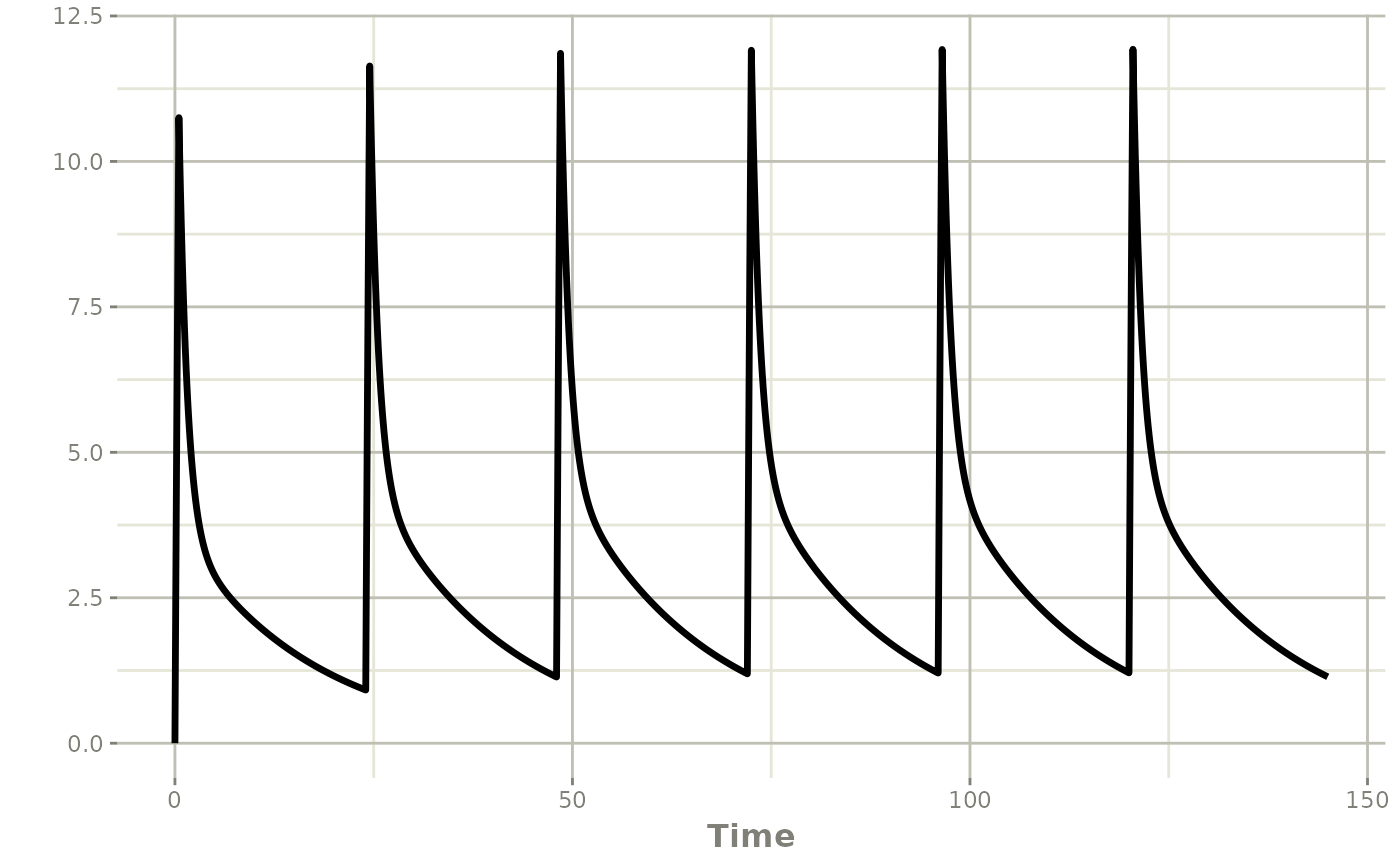
The difference between the cumulative AUC at H144 and that at H120 gives the AUC 0-24 after the last dose. Using data.table is optional, but the syntax is more convenient.
library(data.table)
data.table(map_patient$model)[time==144,AUC] -
data.table(map_patient$model)[time==120,AUC]
#> [1] 72.19084Optimal dose for an intravenous ganciclovir injection
The optimal dose to achieve an AUC of 50 mg.h/L can be determined for
a new injection of IV ganciclovir by setting
cmt_dose = "centr".
poso_dose_auc(patient,mod_ganciclovir_Caldes_2009,tdm=TRUE,
time_dose = 145,
duration = 1,
time_auc = 24,
target_auc = 50,
cmt_dose = "centr")
#> using C compiler: ‘gcc (Ubuntu 13.3.0-6ubuntu2~24.04) 13.3.0’
#> $dose
#> [1] 156.533
#>
#> $type_of_estimate
#> [1] "point estimate"
#>
#> $auc_estimate
#> [1] 50
#>
#> $indiv_param
#> THETA_cl THETA_v1 THETA_cld THETA_v2 THETA_ka THETA_baf add.sd prop.sd
#> 1 7.49 31.9 10.2 32 0.895 0.825 0.465 0.143
#> ETA_cl ETA_v1 ETA_ka ETA_baf covar
#> 1 0.05256373 -0.4773317 -3.099019e-06 2.772041e-07 25Optimal dose for an oral valganciclovir administration
The optimal dose to achieve an AUC of 50 mg.h/L can be determined for
an administration of oral valganciclovir by setting
cmt_dose = "depot".
poso_dose_auc(patient,mod_ganciclovir_Caldes_2009,tdm=TRUE,
time_dose = 145,
time_auc = 24,
target_auc = 50,
cmt_dose = "depot")
#> $dose
#> [1] 193.1298
#>
#> $type_of_estimate
#> [1] "point estimate"
#>
#> $auc_estimate
#> [1] 50
#>
#> $indiv_param
#> THETA_cl THETA_v1 THETA_cld THETA_v2 THETA_ka THETA_baf add.sd prop.sd
#> 1 7.49 31.9 10.2 32 0.895 0.825 0.465 0.143
#> ETA_cl ETA_v1 ETA_ka ETA_baf covar
#> 1 0.05256457 -0.4773339 -9.298227e-07 -1.072357e-07 25Keeping the default value of cmt_dose, which is the first compartment declared in the PK model, would also work here.
Note in this case the estimated dose should be corrected by a factor of 1/0.72 as the Caldès 2009 paper states:
“As NONMEM estimated the pharmacokinetic parameters of ganciclovir, valganciclovir doses were converted to their equivalent ganciclovir content by multiplying the valganciclovir dose by 0.720 (corresponding to the ratio between the molecular weights of ganciclovir and valganciclovir).”